Crystal Clear
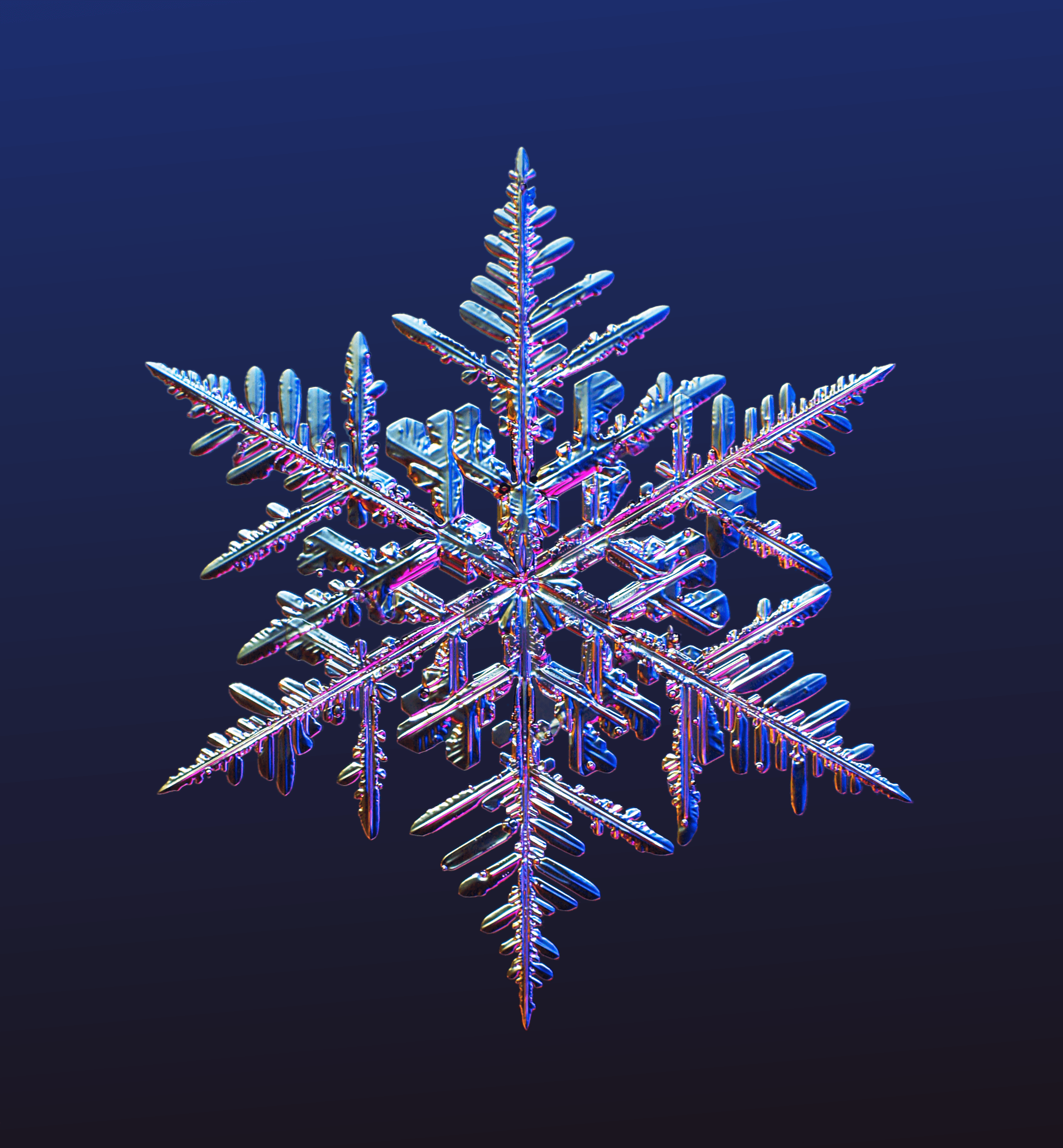
Snowflakes are captivating, whether you’re a powder-hound engrossed with the everchanging snowpack or a kid-at-heart filled with memories of standing outside and looking up, catching softy falling flakes on your tongue. The frozen crystals are a sight to see and, for some, to study.
Ken Libbrecht is a professor of physics at the California Institute of Technology. Originally from North Dakota, Libbrecht has dedicated his work life to studying the molecular dynamics of snow-crystal growth; or, as he calls it, the physics of snowflakes. Early on in his career, he became fascinated with each icy particle’s journey.
“We know an awful lot about water, but people are puzzled about why crystals grow into the shapes they do,” he says. “How do the surfaces grow? How do they accumulate water vapor? How fast does it happen?”
The life of a snowflake, it turns out, is complex. The first thing to clear up is the matter of “snowflake” versus “snow crystal.” A snowflake can be anything from sleet and hail to snow crystals. But the picturesque, uniquely shaped crystals known to all—typically coming in a hexagonal shape—and commonly called snowflakes are actually snow crystals.
To learn more about these dazzling natural marvels, Libbrecht took to photography. He has traveled to many places where the snow falls, from Utah to Alaska, seeking out the perfect temperatures for snow crystal growth. This treasure hunt has produced an immensely mixed bag of shapes, sizes, and other distinctions.
“Since I live in Southern California, it doesn’t work to just go outside,” he says. “I look for places that get a lot of snow, with temperatures that stay pretty cold. As far north as people live, that’s where the best snowflakes are; Fairbanks, Alaska, is one of the coldest places and one of the best for snow crystals.”
Although Teton Valley boasts impressive snow totals year after year, Libbrecht explains that the Tetons aren’t quite cold enough for reliable snow crystal formation (although on frigid January days, some locals might beg to differ). But the amateur seeking a lovely photograph or on the hunt to uncover a specific shape is bound to discover a hodgepodge of crystals during each snowfall here.
Be on the lookout for a brisk winter day, with temperatures of around 5 degrees and little wind. During such conditions, the most recognizable star-shaped crystals, called stellar dendrites, are likely to appear. Dendrite means tree-like, and these crystals boast long branches with varying offshoots and designs.
“The biggest crystals happen at 5 to 10 degrees Fahrenheit; that is the magic temperature,” Libbrecht says. “When it is much warmer, like 23 degrees, you tend to get columned crystals. Once it is below zero, it becomes too cold.”
The creation of a single snowflake in the atmosphere, which takes about forty-five minutes, begins when water vapor converts into ice without becoming rain. A snow crystal is not just a frozen raindrop. That, Libbrecht explains, is sleet or hail. As the crystal falls, a hexagonal plate sprouts six branches that experience the same conditions at the same time, so the growth occurs simultaneously and identically. The shape is determined by the path the crystal takes while falling through the clouds. A close examination of a single crystal reveals a mesmerizing and natural work of art.
Libbrecht offers this advice for those wanting to explore the universe of snow crystals: “First go out, walk around, and see what you can find. Bring a magnifying glass and see what you can see,” he says. “It is fascinating what you’ll discover. Often you will see many different shapes: flowerlike crystals, fernlike stellar dendrites, and columns that have two plates on different ends. There is a whole menagerie of crystals.”
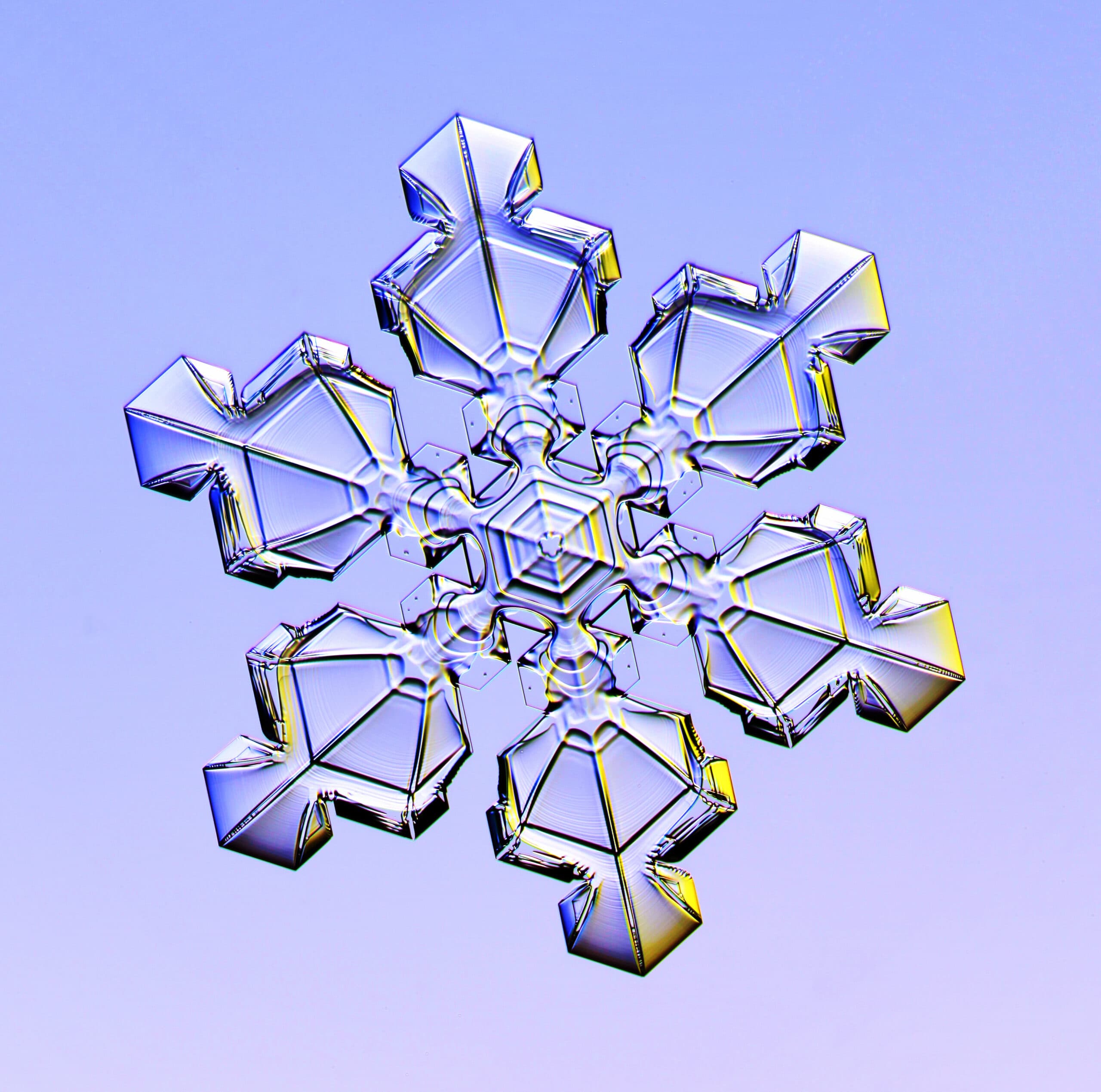
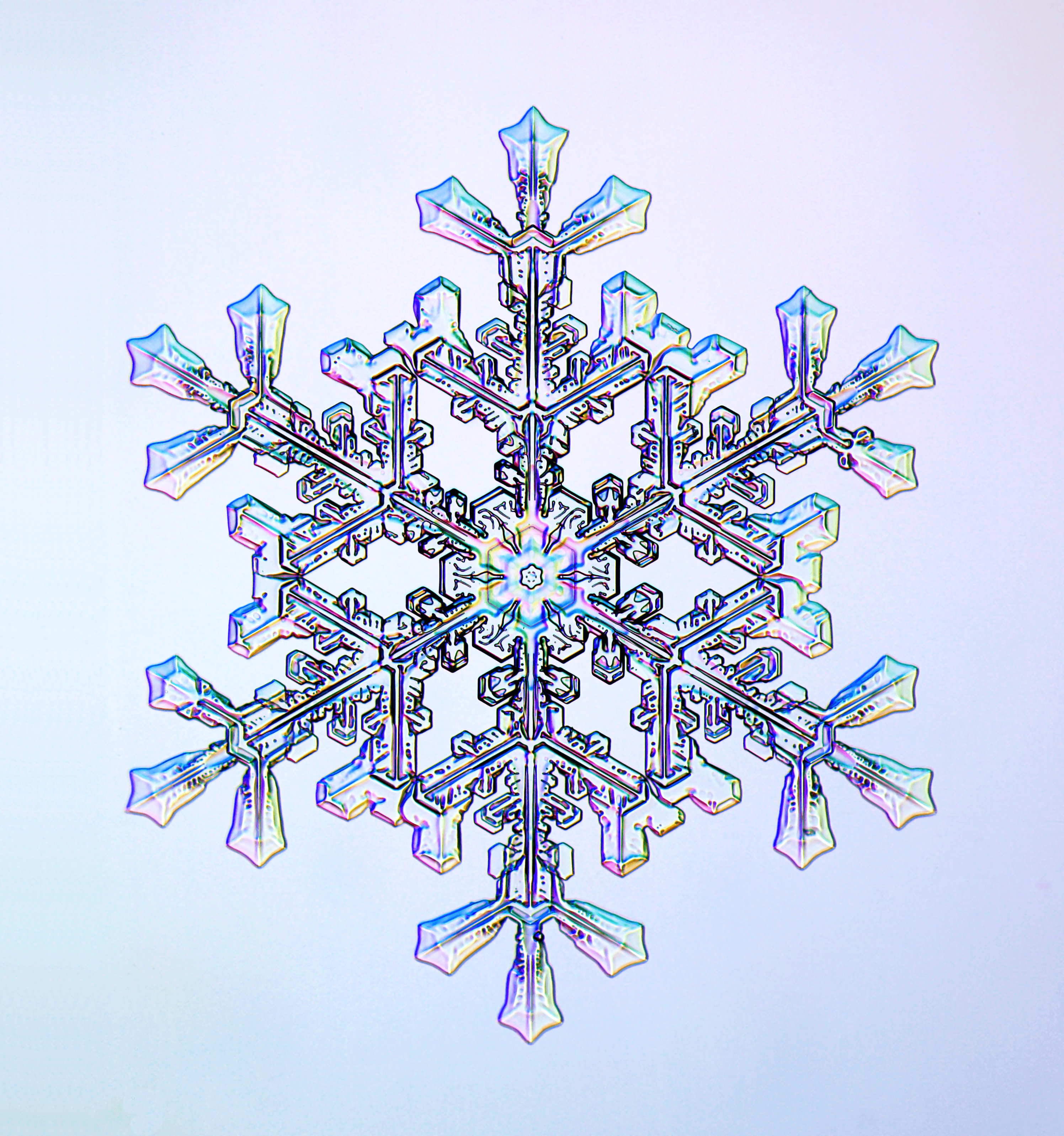

Libbrecht remembers the long, cold winters in North Dakota of his childhood as a bit monotonous. Discovering the excitement of searching for new snow crystals spiced up his otherwise dull days and fueled his love of science.
“If you stop and think about it, there is a lot of cool stuff going around you all the time,” he says.
Interested in photographing the minute icy wonders? Libbrecht recommends grabbing a three-dollar magnifying glass, a piece of black cloth, and your camera phone. Or, just head out with the camera and see what lands on your (dark-colored) sleeve. For his professional photographic escapades, however, the system is a bit more thorough.
“I am always looking for more detail; that’s the scientist in me,” he says.
First, Libbrecht lets the crystals fall on blue foam core. The crystals start to change almost immediately and begin to lose their nice shape quickly, so he races the clock.
“After I find some nice crystals, I use a little tiny paintbrush to move it. The bristles have tiny burs on them, so it’s easy to pick up a snowflake,” he says. “I then drop it on a microscope slide and quickly take a photograph. The whole process takes a minute. You must go fast.”
Libbrecht has turned his photography into several books, including a field guide to identifying different crystal formations. Now, he has over 10,000 photographs to study, but there are always more crystals to discover.
At some point in your childhood, you were probably told, “No two snowflakes are exactly alike.” In nature, this is accurate. “In the outside world, the growth of the crystal depends on the environment around it,” Libbrecht says. “The crystal grows as it is blowing around. Every time the temperature and humidity change around it, it grows differently.”
The final shape of the crystal depends on the path it follows, which can be very complex. Since no two crystals follow the exact same path, no two look exactly alike. But in the lab, Libbrecht can grow identical crystals.
“It is fun in the sense that bird watching is fun,” Libbrecht says. “You don’t want to watch other people doing it, but if you are into it, it is a wonderful experience.”




